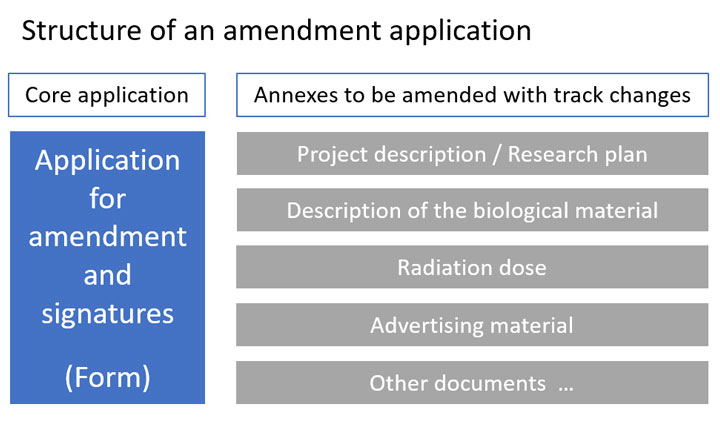
Application for amendment
The application for changes – or amendment – of an already approved ethical application must be made via the Ethics Review Authority’s application portal ETHIX. The application has to be completed in Swedish with the Swedish documents.
ETHIX: Ethics Review Authority’s application portal ETHIX
Information and translation in English of the Swedish documents can be found on the webpage of the Ethics Review Authority . The English documents are only for guidance.
The application must be signed by the principal investigator and the dean of school.
A significant change is typically altering the security of the participants, or the risk-benefit balance. Examples of significant changes that may require an amendment application:
- Change of research principal or principal investigator.
- Many more research samples/subjects/participants should be included.
- New units or study places should be involved.
- New methods or new analyses should be performed on material already collected (under the same main issue).
If a change to an original project is extensive, such as a new study approach or study hypothesis, new groups of research samples/subjects/participants with characteristics other than the original ones, a completely new application may need to be done.