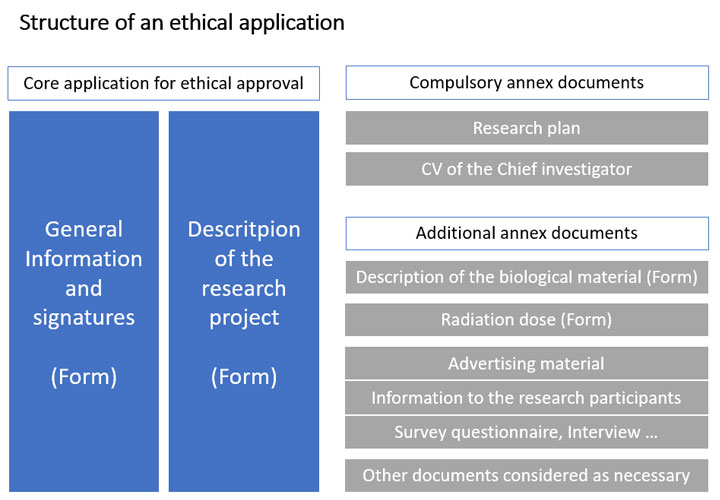
Application for ethics review
The application for an ethics review regarding research on humans (including human participants, biological samples or sensitive personal data) must be made via the Ethics Review Authority’s application portal ETHIX. The application has to be completed in Swedish with the Swedish documents.
Ethics Review Authority’s application portal ETHIX
Information and translation in English of the Swedish documents can be found on the webpage of the the Ethics Review Authority . The documents in English are only for guidance.
The application must be signed by the principal investigator and the dean of school. In collaborations, one research principal must be appointed to be responsible for the ethics review application.